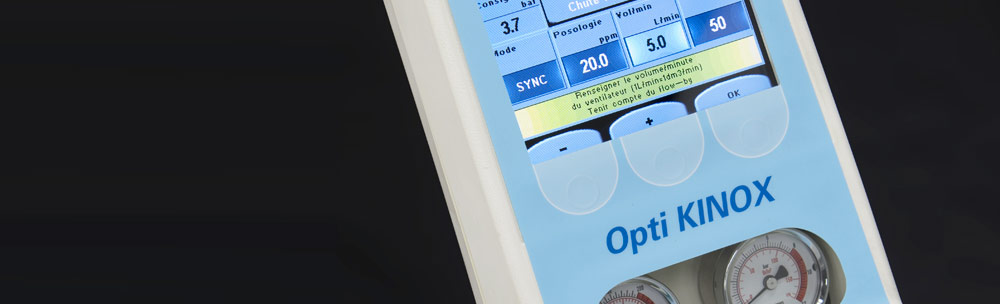
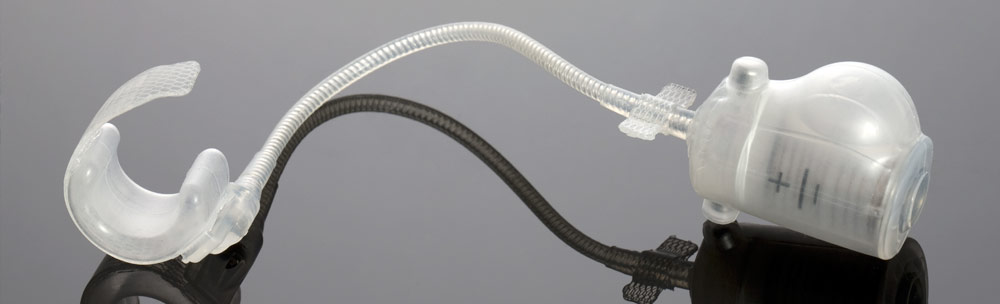
Active medical DEVICEs
STATICE is closely involved and committed to the production of regulated class I, II, and III electro-medical devices.
Our R&D team's expertise is essential for the complete development of any project, and includes:
- Precision micro-technical skills
- Piloting electronics (captors and activating technology)
- Development of incorporated software
- Development of man/machine interface systems.
The R&D department at STATICE will enable you to benefit from their continuous research into thermal regulation, UV emissions, micro-fluids, micro-outputs for gas, and integration of MEMS (Micro-Electro-Mechanical Systems)...
In applying the current regulations, CEI 60601, ISO 62304, and reference 93/42/CE, STATICE now finds itself in operating rooms, ICU wards, and in GP surgeries.